REMOVING BARRIERS TO LOW DOSE CT LUNG SCREENING PROGRAMS
According to a recent article “Imaging Administrators: The Overlooked Barrier to Lung Cancer Screening Implementation”, Buehler, et.al, July 8, 2021, LDCT screening may just need to get a bit more support from those who are responsible for its practical implementation; Imaging Directors.
Researchers performing the study conducted a phone interview survey of administrators and lung cancer program coordinators at 76 imaging sites that provide low-dose CT exams for lung cancer screening. Of the 76 sites included in the survey, only eight reported having no barriers to lung cancer screening.
The survey was conducted shortly after a lung cancer screening guidance update released in March by the U.S. Preventive Services Task Force (USPSTF) that broadened the pool of people eligible for screening by lowering the starting age to 50 and adjusting smoking history to 20 pack years.
This is not entirely unexpected as diagnostic imaging centers are not designed for routine screening applications. Typically patients show up to the MR or CT suite, have an imaging procedure completed, the study is interpreted, report dictated and then delivered to the referring physician. The referring physician has typically had the responsibility of communicating directly with the patient, and scheduling follow on imaging as appropriate to the treatment regimen.
LDCT screening is not just a new procedure in diagnostic imaging, it’s a whole new paradigm requiring scheduling of follow up exams, delivery of result letters to the patients (in addition to the physicians), providing recall letters if appropriate, accounting for, documenting and reporting incidental findings and an upload to a national registry for reporting and reimbursement. Additionally, there is a patient intake screening and eligibility questionnaire that must be completed in advance to make sure the patient is qualified to participate in the screening and for the clinic to be reimbursed. What’s more, there is a relatively new reporting paradigm that must be followed by the physicians, put in place by the ACR, namely LungRADS™. In the final analysis LDCT screening has more in common with mammography screening than routine diagnostic imaging.
Perhaps these reasons account for manufacturers of Mammography Analytics Software packages being the first to come out with LDCT screening, analytics, and workflow packages and recently the first to include COVID-19 tracing functionality. In a recent review of one such manufacturers web site (PenRad.com manufacturers of PenLung*) I found the following partial list of LDCT Lung Software features that are called out; an uncanny resemblance to those required for mammography screening.
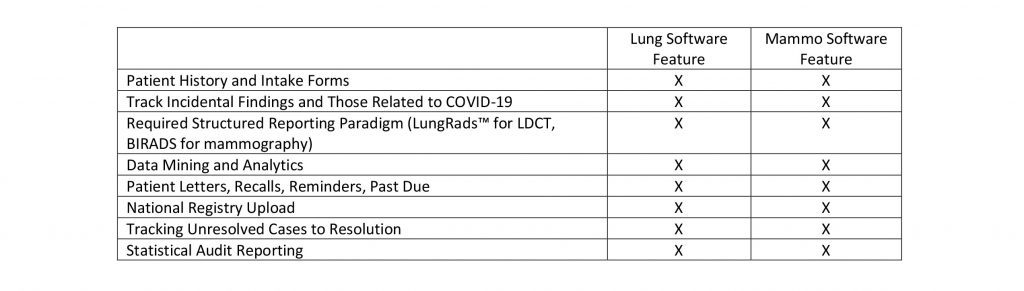
Mammography Analytics Software vendors have been relatively successful in selling their software programs to participating LDCT screening centers but there are still many more that are struggling with keeping up with all the added requirements. It is not surprising in the least, that busy Imaging Directors have not found the time or the enthusiasm to really get behind LDCT screening programs and roll them out to the public and base of referring physicians.
In a recent interview with Melissa Kramps, Lung Cancer Program Coordinator at a major metropolitan healthcare organization, Melissa explained, “Prior to going live with PenLung the administrative tasks associated with our LDCT Lung Screening Program was a huge headache. We had to manually enter patient names, addresses, physician names, addresses, and manually print each letter; we had to manually upload all patients and results to the national lung screening registry. The whole process was time consuming and tedious. When we got started with PenLung it was like a breath of fresh air. All these administrative tasks were automated, our workflow improved significantly, and our program is thriving. PenRad makes the whole administrative process easy.”
My recommendation to the Imaging Director interested in starting or supplementing an already existing LDCT screening program is to go talk with their counterpart in women’s imaging or call one of the handful of Mammography Analytics Software providers. Much of the work has already been done and by following their lead you might just find out an LDCT program is straightforward to implement, not terribly expensive and immensely rewarding.
Click to download this case study
*About PenLung (from www.penrad.com)
Lung screening presents several challenges for diagnostic imaging centers offering Low Dose Computed Tomography (LDCT). Patients must be screened for eligibility requirements, and participating centers are required to upload pertinent exam information to a lung cancer screening registry to qualify for payment. PenLung offers diagnostic imaging centers a comprehensive unified lung screening and tracking software solution to manage patients participating in lung screening programs. This system also provides automated web and tablet calculators to collect smoking and environmental risk history for patient eligibility, along with the facility’s LungRADS™ reporting combinations, which in turn automates the entire reporting, auditing, and lung cancer screening registry upload requirements.
About the author
About the author. Daniel D. Bickford (www.linkedin.com/in/daniel-bickford) is President of Pintail Strategic Consulting which has provided sales and marketing services to the diagnostic imaging industry, since 2015. PenRad is a current client of Pintail Strategic Consulting. Daniel was co-founder of Confirma Inc., the pioneer of breast MRI CAD technology and manufacturer or of CADstream.